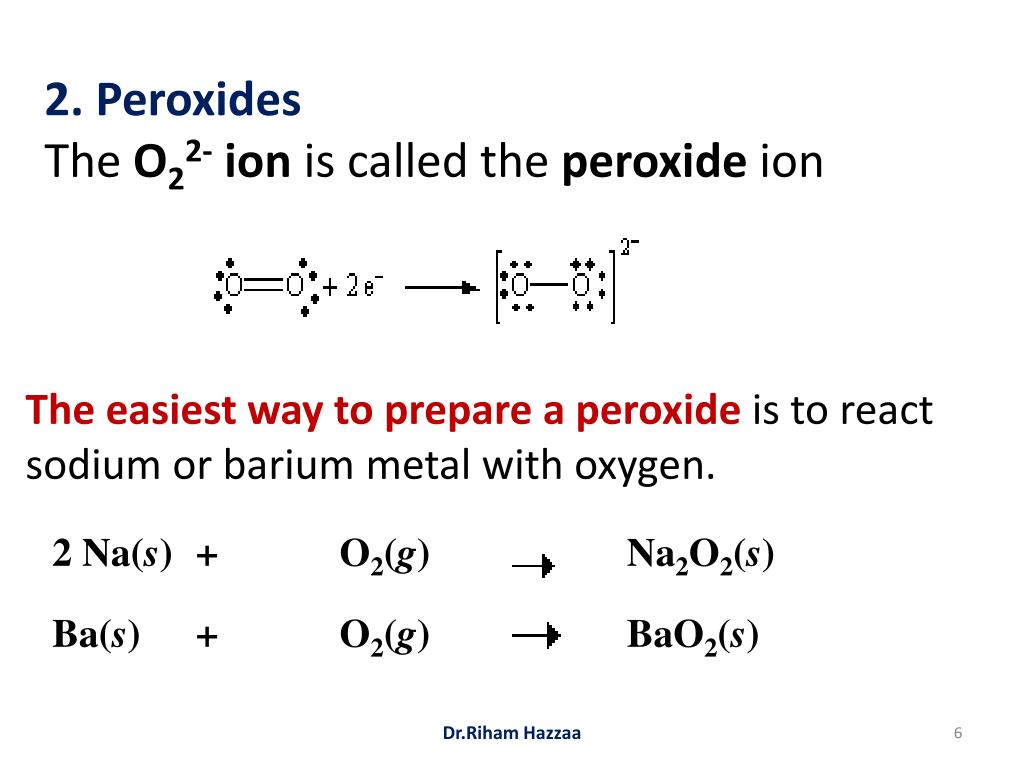
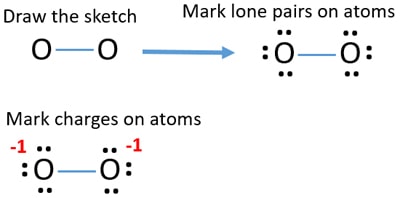
Thus the Lewis structure of the peroxide ion with the formal charges is as follows Step 10 of 34 As a single bond exists between two oxygen atoms so the bond length will be m (refer to table in the textbook) Step 11 of 34Chemistry, 24 1936 gamer0078 How many nonbonding electron pairs are there in the lewis structure of the peroxide ionOxygen Lewis structure of oxygen Preparation of Oxygen a Thermal decomposition of potassium chlorate(V) 2 KClO3 ( s ) 2 KCl ( s ) 3O2 ( g ) →

Molecule Definition Examples Structures Facts Britannica
Superoxide ion lewis structure
Superoxide ion lewis structure-Course Title CH 301;Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO −It is an unstable structural isomer of nitrate, NO − 3Although its conjugate acid peroxynitrous acid is highly reactive, peroxynitrite is stable in basic solutions It is prepared by the reaction of hydrogen peroxide with nitrite H 2 O 2 NO − 2 → ONOO − H 2 O



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube
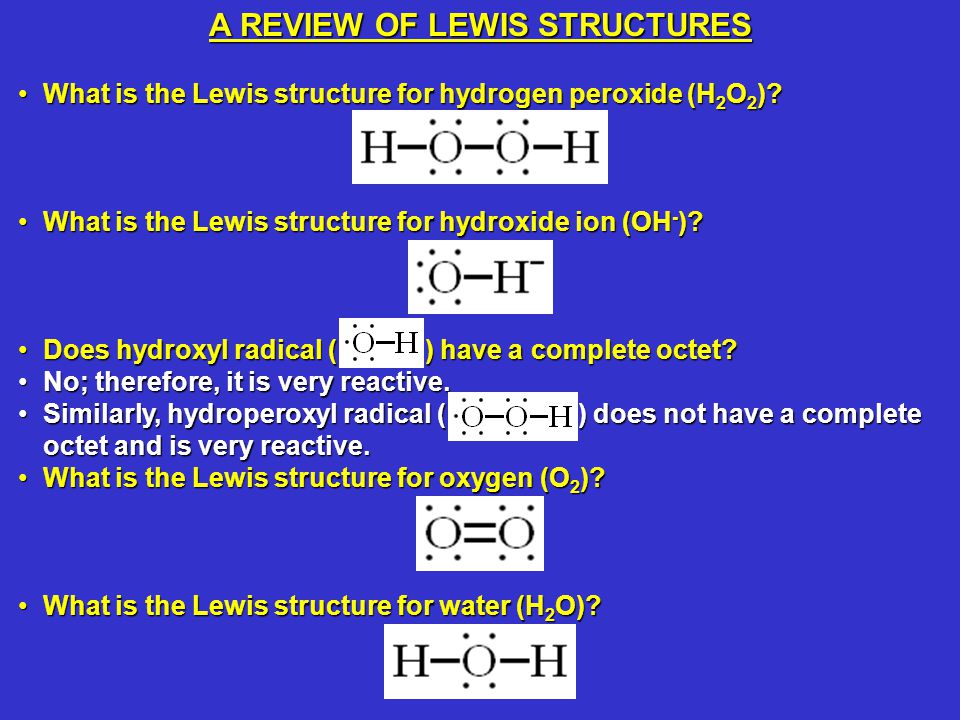
Type Notes Uploaded By adibamobin Pages 2 This preview shows page 1 2 out of 2 pagesIn order to draw the Lewis structure of Peroxide ion O 2 2we just have to know how many free electrons there will be This is calculated knowing that for every atom of oxygen it has 6 electrons and sum the 2 electrons that the ion itself has The electrons would be 2 (6) 2 = 1 4 H2O2 lewis structure contains two OH bond and one OO bond connected with a single bond Also 4 total lone pairs present in the lewis structure of H2 The hydrogen peroxide lewis diagram is very simple and the procedure for drawing it same as the other molecules Let's see how to draw this step by step
🔴 Answer 1 🔴 on a question How many nonbonding electron pairs are there in the lewis structure of the peroxide ion, o22−?Hydrogen peroxide, H2O2, the oxygen atoms are in the center (H–O–O–H) 7 In drawing Lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms EXAMPLE Write the Lewis structure for CH2O where carbon is the centralNov 30, 18 · Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol Transcript This is the O2 2 Lewis structure For the peroxide ion, Oxygen has six valence electrons We have two Oxygens, and then we need to take in account these extra two valence electrons up here, so we'll just add them for a total
The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has aVideo Drawing the Lewis Structure for H 3 O For the H3O Lewis structure we first count the valence electrons for the H3O molecule using the periodic table Once we know how many valence electrons there are in H3O we can distribute them around the central atom and attempt to fill the outer shells of each atomSee the cyanide ion for an example The above, in addition to keeping in mind that a carboxylic acid is a Bronsted/Lowry acid ie proton donor should enable the construction of an acceptable Lewis structure


Making Molecules Lewis Structures And Molecular Geometries Annenberg Learner


Ppt Quize 2 Define Ionization Energy Write Trends Of Ionization Energy In Periodic Table And Why Powerpoint Presentation Id
Structural Formula O 2 2 Peroxide IonOur videos will help you understand concepts, solve your homework, and do great on your examsLewis Structure Chlorite Ion ClO 2Lewis Structure Chlorate Ion ClO 3Lewis Structure Perchlorate Ion ClO 4Lewis Structure Carbon Dioxide CO 2 Lewis Structure Carbonate Ion CO 3 2Lewis Structure Sulfuric Acid H 2 SO 4 Lewis Structure Phosphoric Acid H 3 PO 4 Lewis Structure Azide Ion N 3Lewis Structure Hydroxyl Amine NH


4 General Organic And Biochemistry 8 E Bettelheim



O2 2 Lewis Structure How To Draw The Lewis Structure For O2 2 Youtube
See Answer Check out a sample Q&A hereJul 22, 14 · Carboanions also exist;The answers to answerhelpercom


Lecture Presentation Chapter 8 Basic Concepts Of Chemical



Sample Exercise 8 1 Magnitude Of Lattice Energies Ppt Video Online Download
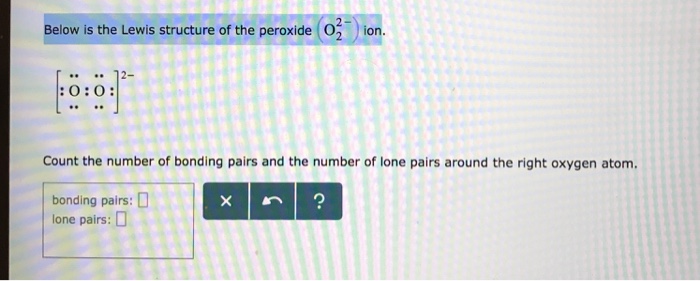
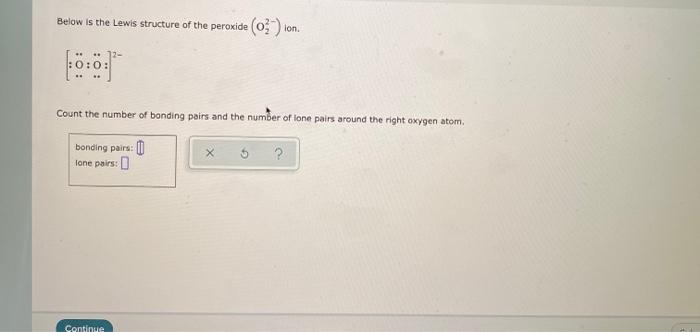
Below is the Lewis structure of the peroxide 0 ion 12 00 Count the number of bonding pairs and the number of lone pairs around the right oxygen atom bonding pairs lone pairsHydrogenperoxide(1) HO2 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activitiesSulfate ion, SO 4 2, is an example of a highvalent sulfur compound Sulfur, like oxygen, frequently forms two bonds In sulfate, the sulfur is attached to four different atoms We could draw that structure in two ways A structure that obeys the octet rule would have a single bond to each oxygen That would satisfy the octet of sulfur



Draw A Lewis Dot Structure Of D2o2 Novocom Top



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube
See the Big List of Lewis Structures Transcript This is the O2 2 Lewis structure For the peroxide ion, Oxygen has six valence electrons We have two Oxygens, and then we need to take in account these extra two valence electrons up here, so we'll just add them for a total of 14 valence electrons for the 02 2 Lewis structureOther articles where Peroxide ion is discussed oxide Peroxides The peroxide ion, O22−, has a single oxygenoxygen covalent bond and an oxidation state of −1 on the oxygen atoms The peroxide ion is a powerful hydrogen ion acceptor, making the peroxides of the alkali metals and alkaline earth metals strong bases Solutions of these peroxidesA stepbystep explanation of how to draw the O2 2 Lewis Structure (Peroxide Ion) Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO −It is an unstable structural isomer of nitrate, NO − 3Although its conjugate acid peroxynitrous acid is highly reactive, peroxynitrite is stable in basic solutions



The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Gas Molecules Tech Company Logos
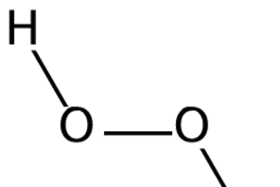


Hydrogen Peroxide Soundbite Rsc Education
(a) 7 (b) 6 (c) 5 (d) 4 (e) 3 check_circle Expert Answer Want to see the stepbystep answer?Nov 05, · In the sulfite ion, SO 3 2– for example, the oxidation number of sulfur is 4, suggesting that only four sulfur electrons are involved in the bonding Since sulfur has six valence electrons, we conclude that two electrons are not involved in the bonding, ie, that there is a lone pair With this clue, a plausible Lewis structure is muchBenzoyl Peroxide is a peroxide with antibacterial, irritant, keratolytic, comedolytic, and antiinflammatory activity Upon topical application, benzoyl peroxide decomposes to release oxygen which is lethal to the bacteria Proprionibacterium acnes Due to its irritant effect, benzoyl peroxide increases turnover rate of epithelial cells, thereby peeling the skin and promoting the resolution
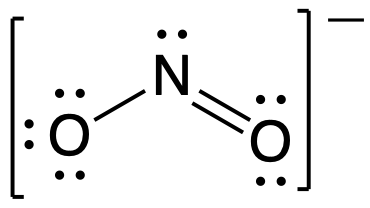


Resonance Structures And Formal Charge M8q3 Uw Madison Chemistry 103 104 Resource Book


Sodium Peroxide Na2o2 Chemspider
Jan 08, 18 · Main Difference – Peroxide vs Superoxide An oxide is any chemical compound that contains one or more oxygen atoms Oxides can be oxides containing oxide anions (O 2), peroxides containing peroxide anions (O –) or superoxides containing superoxide anion (O 2 –)A peroxide is any compound that is composed of an oxygenoxygen single bondThis can be either in the formO 2 2(peroxide ion) Lewis Structure O 2 2(peroxide ion) anion contains only two oxygen atoms Peroxide anion has 2 charge In O 2 2lewis structure, each oxygen atom has 1 charge and three lone pairs Both oxygen atoms are joint through a single bond In this tutorial, we are going to draw the lewis structure of O 2 2ion step by stepFullscreen check_circle Expert Answer Want to see the stepbystep answer?



Peroxide And 2 Extra Electrons Chemistry Stack Exchange



Chapter 8 Concepts Of Chemical Bonding Ppt Download
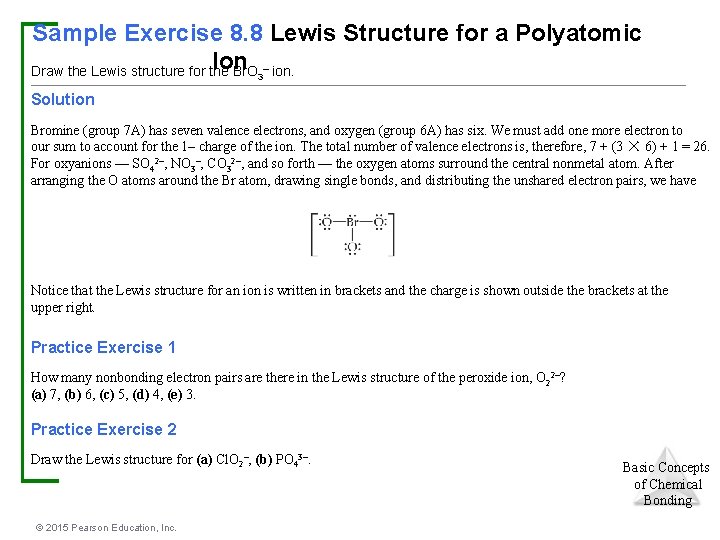
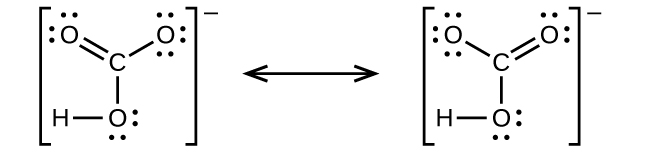
When the Lewis structure of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets For example, consider the ammonium ion, NH 4 , which contains 9 (5 from N and 1How many lone pairs are in the Lewis Dot structure for the peroxide ion O 2 2 1 How many lone pairs are in the lewis dot structure School University of Texas;In a Lewis structure, formal charges can be assigned to each atom by treating each bond as if onehalf of the electrons are assigned to each atom Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate Determine the formal charge of each element in the following (a) HCl
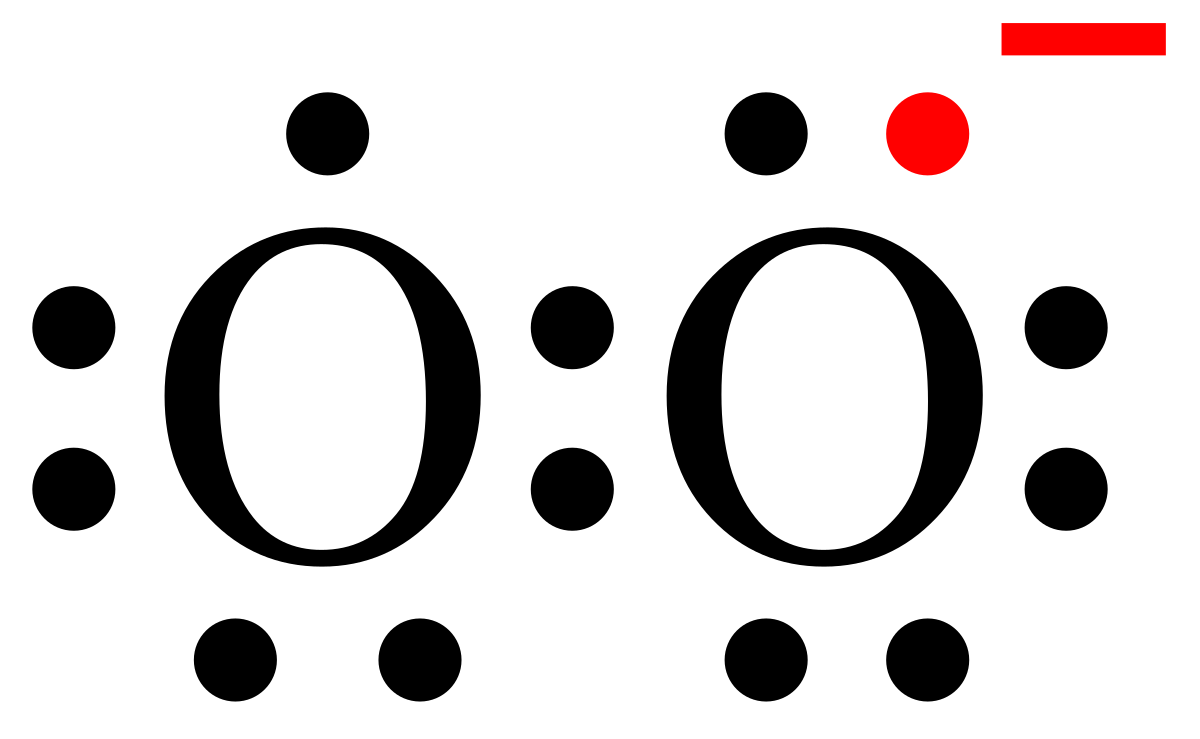


Superoxide Wikipedia



Lewis Electron Dot Structures Chemistry For Non Majors
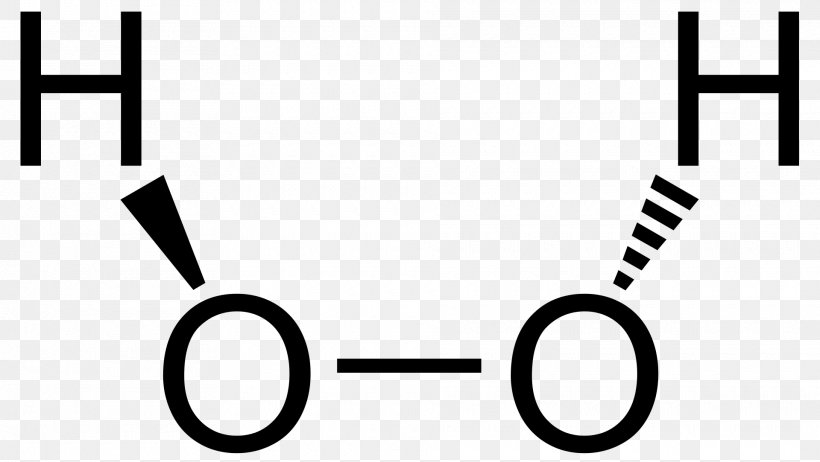
Dec 19, 12 · In the ammonium ion, nitrogen has lost one of its electrons and therefore has a positive charge of 1 H 3 O hydronium cation In this Lewis structure of the hydronium ion, oxygen is sharing 3 of its valence electrons in covalent bonds with hydrogen and there is a single pair of unshared electrons 3 2 = 5The Lewis structure of hydrogen peroxide contains an OO single bond, as shown in the figure below The VSEPR theory predicts that the geometry around each oxygen atom in H 2 O 2 should be bent But this theory cannot predict whether the four atoms should lie in the same plane or whether the molecule should be visualized as lying in twoConstruct a Lewis Dot Structure by Bob Hanson back to the General Chemistry ToolkitGeneral Chemistry Toolkit


Solved Below Is The Lewis Structure Of The Peroxide 0 Ion Chegg Com



Construct A Lewis Structure For Hydrogen Peroxide H2o2 In Which Each Atom Achieves An Octet Of Brainly Com
Answer to Below is the Lewis structure of the peroxide (O2^2) ion Count the number of bonding pairs and the number of lone pairs around the left oxygen atomA superoxide is a compound that contains the superoxide ion, which has the chemical formula O− 2 The systematic name of the anion is dioxide The reactive oxygen ion superoxide is particularly important as the product of the oneelectron reduction of dioxygen O2, which occurs widely in nature Molecular oxygen is a diradical containing two unpaired electrons, and superoxide resultsAfter writing the structure, the entire structure should then be placed in brackets with the charge on the outside of the brackets at the upper right corner Example Write the Lewis structure for the ammonium ion (NH 4 ) Answer Hydrogen atoms are always placed on the outside of the molecule, so nitrogen should be the central atom


O2 2 Peroxide Ion Lewis Structure



H2 Lewis Structure Novocom Top
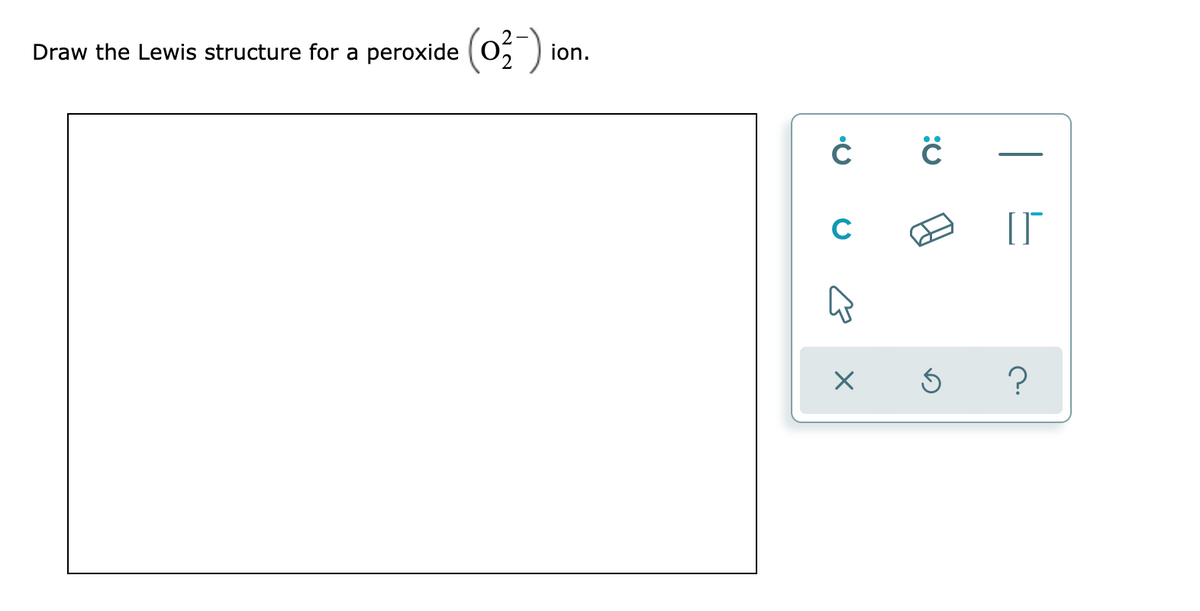
Question help_outline Image Transcriptionclose Draw the Lewis structure for a peroxide (0,) ion C ?Our videos prepare you to succeed in your college classes Let us help you simplify your studying If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back!Dec 11, 15 · The structure you have in mind is the diatomic oxygen molecule, with 12 electrons in its system overall While the peroxide ion overall has 14 electrons in its system, since each oxygen has 6 valence electrons and the system also has two
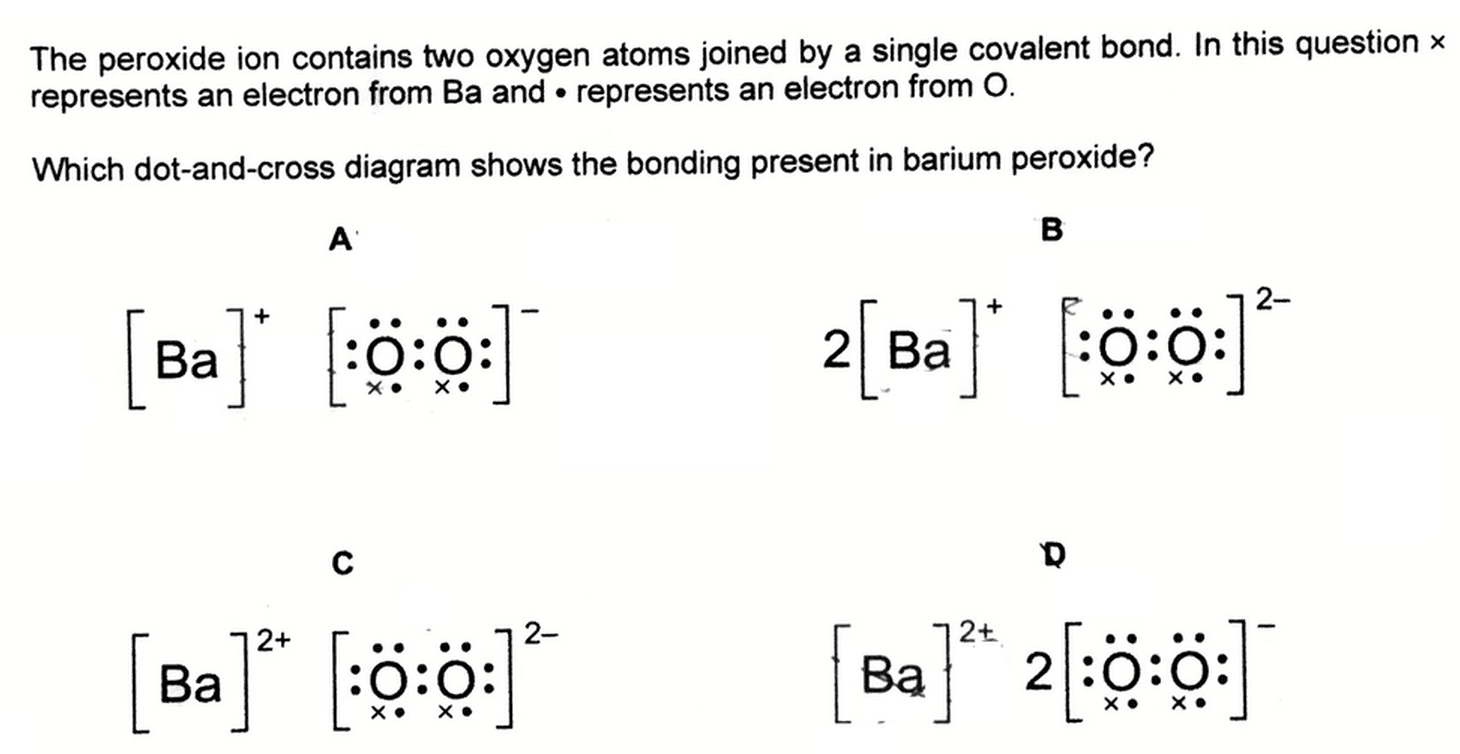


Barium Peroxide Dot Cross Diagram



Oneclass Draw Lewis Electron Dot Structures For Ch3cl Methyl Chloride A Topical Anesthetic H2o2
How many nonbonding electron pairs are there in the Lewis structure of the peroxide ion, O2 2?This is the Lewisdot structure of hydrogen peroxide Here you have two oxygen atoms and the overall required electrons are 6*22=14,An atom of an element is three times as heavier as the mass of an atom of carbon12See Answer Check out a sample Q&A here Want to see this answer and more?


Oxygen Free Radical Electron Dot Diagram


Answered Draw The Lewis Structure For A Peroxide Bartleby
(Hydronium Ion) H 2 O 2 (Hydrogen Peroxide or Dihydrogen Dioxide) HBr (Hydrogen Bromide or Hydrobromic Acid) HF (Hydrogen Fluoride or Hydrofluoric Acid) HCCH (Ethyne) HCl (Hydrogen Chloride or Hydrochloric Acid) HCO 2(Formate Ion) HCO 3(Hydrogen Carbonate Ion or Bicarbonate Ion) HCOOH (Methanoic Acid or Formic Acid) HIUsing Formal Charge to Predict Molecular Structure The arrangement of atoms in a molecule or ion is called its molecular structureIn many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure—different multiple bond and lonepair electron placements or different arrangements of atoms, for instanceThe Lewis structure gives us meaningful information about the bonds between atoms, but Lewis structures do not depict how the molecule exists in threedimensions Depicted on the left in Figure 1 is the Lewis structure for methane (CH 4) showing the central carbon atom singly bonded to four hydrogen atoms Figure 1


Resonance Structures And Formal Charge M8q3 Uw Madison Chemistry 103 104 Resource Book


Hydrogen Peroxide Molecule Of The Month September 06 Html Version
Consider the oxalate ion, C_2O_4^{2} a Draw the Lewis structure, including all equivalent resonance structures b Are all carbonoxygen bonds the same in this ion?Quiz your students on O2 2 Peroxide Ion Lewis Structure using our fun classroom quiz game Quizalize and personalize your teachingDraw the Lewis structure for a peroxide (0,) ion C ?



Chapter 5 Goals Major Goals Of Chapter 5 1 Finding The Exact Location For Valence Electrons Outermost Electrons 2 Discuss The Octet Rule And Why 8 Is A Magic Number When Draw Lewis Dots 3



Draw The Lewis Dot Structure Of O 2 2 Is Snapsolve
70 More Lewis Dot Structures Element number 8 and a member of the Chalcogen Familyor Group 16 of the periodic table From http//enwikipediaorg/wiki/PeroxideThe peroxide ion contains two electrons more than the oxygen molecule These two electrons, according to the molecular orbital theory, complete the two π* antibonding orbitals
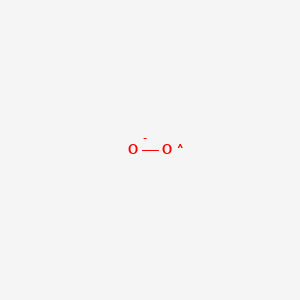


Superoxide O2 Pubchem
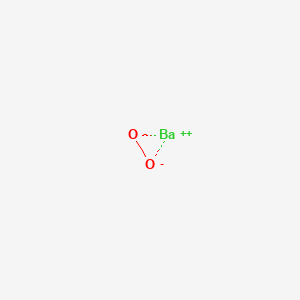


Barium Peroxide Bao2 Pubchem



Iron Ii Oxalate Potassium Ferrioxalate Water Minerals Angle Electronics Png Pngegg
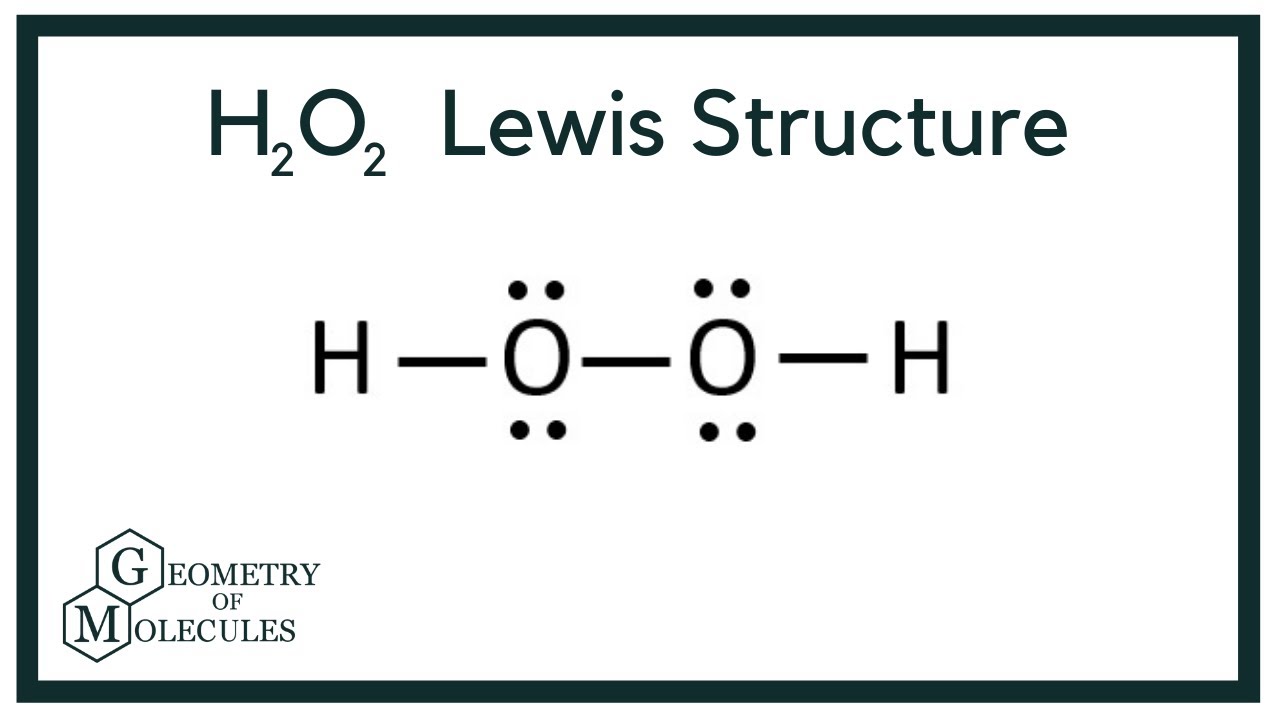


H2o2 Lewis Structure Hydrogen Peroxide Youtube



Peroxide Ion O2 2 Lewis Dot Structure Youtube


Free Radicals And Reactive Oxygen
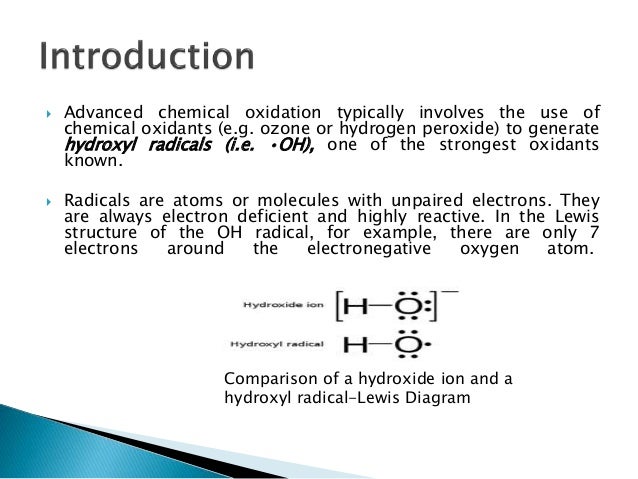


Aop



What Is The Charge Of Peroxide



Lewis Structure Of O2 2 Peroxide Ion Youtube


Solved Below Is The Lewis Structure Of The Peroxide 03 Chegg Com



4 3 Formal Charge And Oxidation State Chemistry Libretexts



Molecule Definition Examples Structures Facts Britannica



Lewis Dot Structure Of O2 Molecule Novocom Top


Solved Oxygen Forms Three Types Of Ionic Compound



Lewis Dot Structure For The Peroxide Ion O2 2 Novocom Top



Mo 0 2 Drawing A Lewis Structure Of Oxygen Superoxide Peroxide Youtube


Lewis Dot Structure Mega Worksheet


Formal Charges And Resonance Chemistry 2e
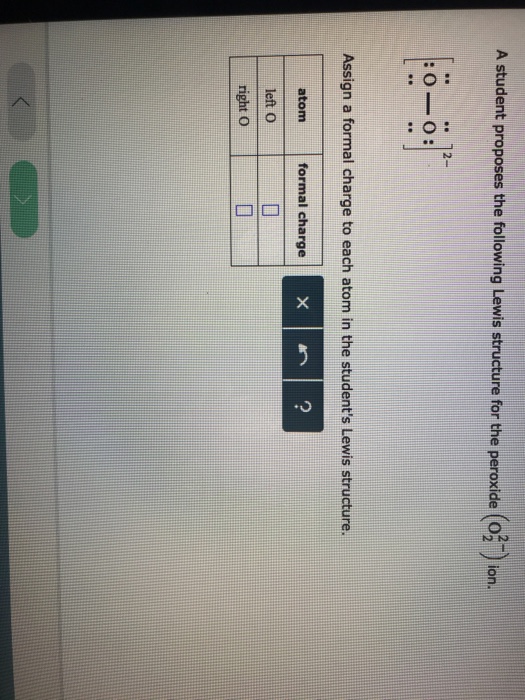


Solved A Student Proposes The Following Lewis Structure F Chegg Com



Explain The Following The Peroxide Ion O2 Clutch Prep



H2o2 Lewis Dot Novocom Top
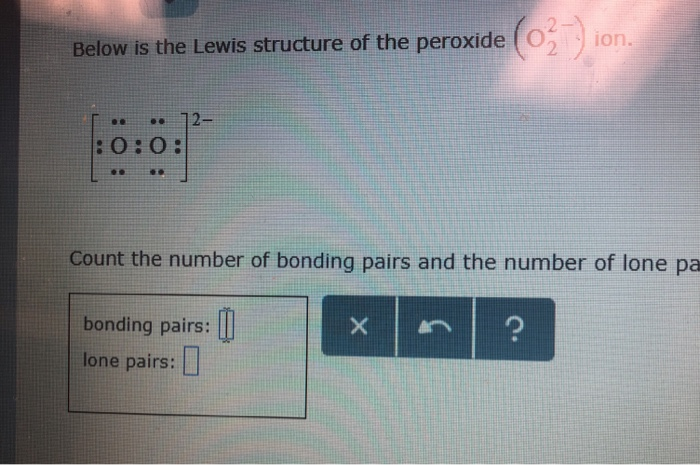


Solved Below Is The Lewis Structure Of The Peroxide O Io Chegg Com
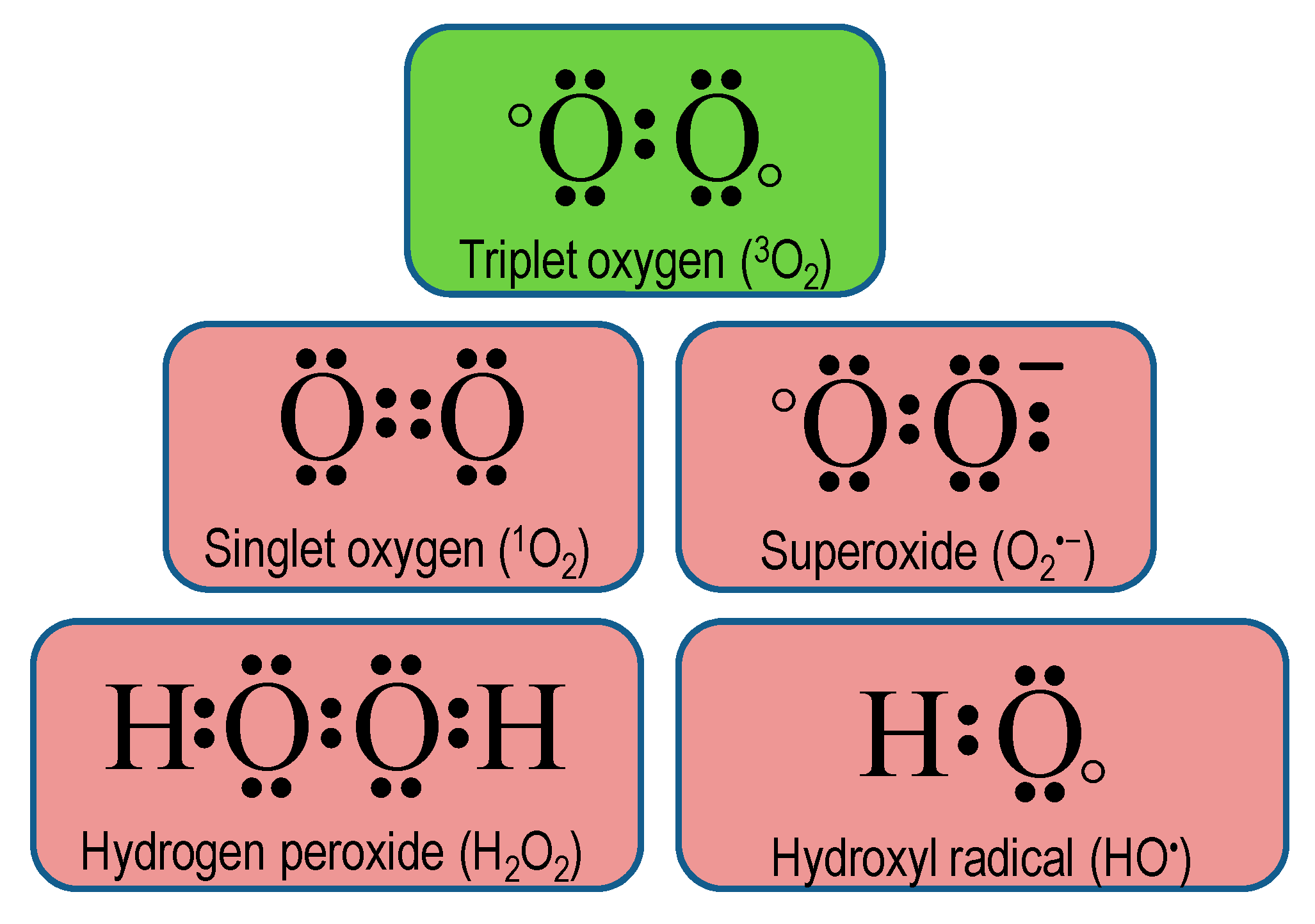


Antioxidants Free Full Text Reactive Oxygen Species And Antioxidant Defense In Plants Under Abiotic Stress Revisiting The Crucial Role Of A Universal Defense Regulator Html


Difference Between Peroxide And Superoxide Definition Structure Examples Differences



Does Peroxide Ion Have Any Unpaired Electrons Chemistry Stack Exchange



Hydroperoxyl Wikipedia



Super Oxide Ion Is Chemistry Questions



Reduction Of O2 To H2o And Its Free Radical Intermediates A Lewis Download Scientific Diagram
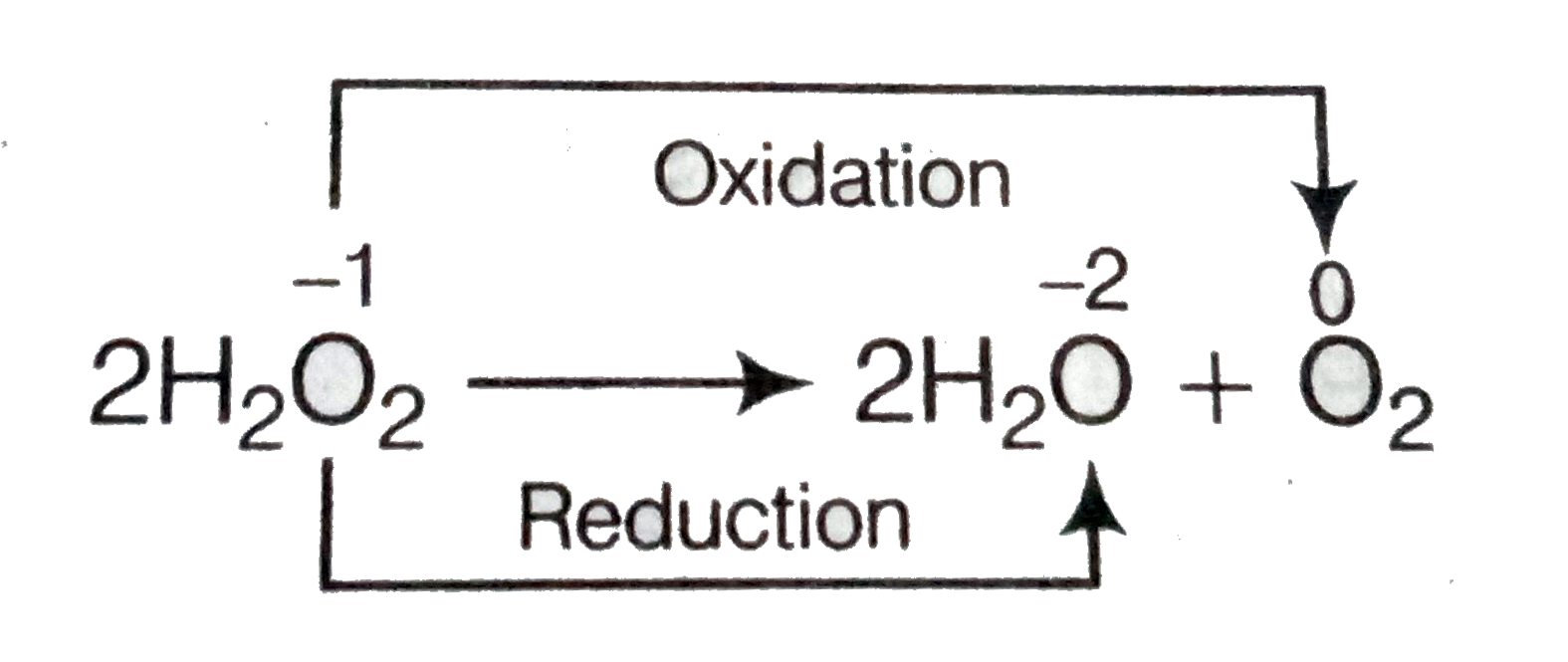


Assertion A The Decomposition Of Hydrogen Peroxide To Form Water



Draw The Lewis Structure For No Clutch Prep



Lewis Dot Structure Of O2 2 Peroxide Ion Youtube



Lewis Dot O2 Molecule Novocom Top


Dioxidanide Ho2 Chemspider


Peroxide Wikipedia


Superoxide O2 Chemspider



An Introduction To Reactive Oxygen Species Measurement Of Ros In Cells April 7 21


Lewis Structure Of Sodium Peroxide Cad Vigyan


Ch 103 Percent Hydrogen Peroxide Ppt Video Online Download



Sample Exercise 8 1 Magnitudes Of Lattice Energies Ppt Download
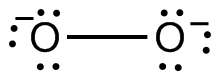


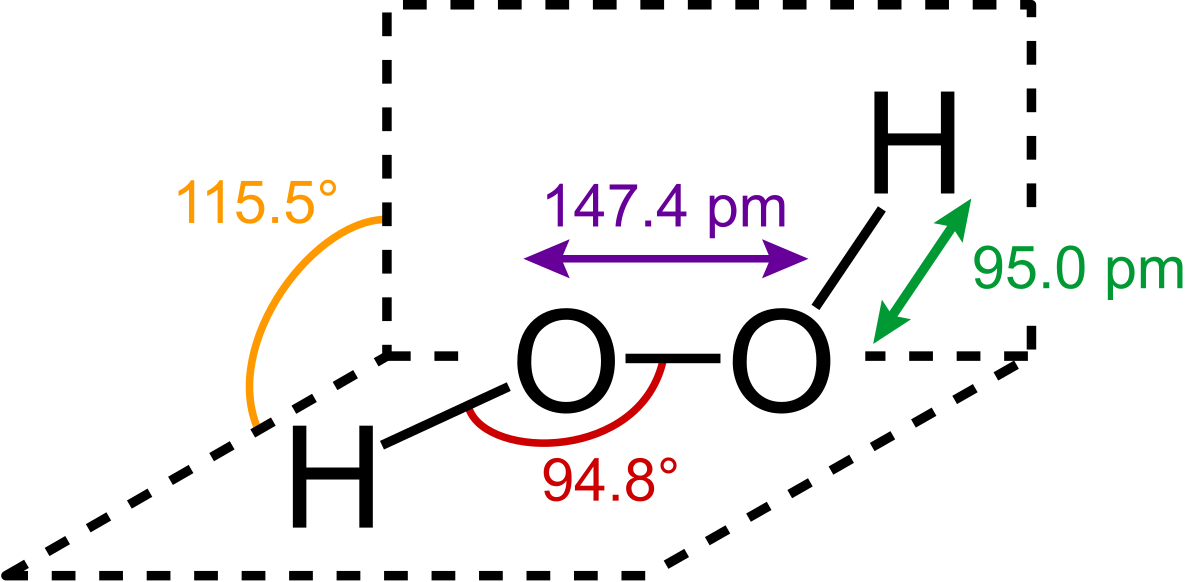
18 2 Group 1a Metals Chemistry Libretexts



What Is The Electron Dot Structure Of Math Ko 2 Math Potassium Superoxide Quora


Hydrogen Peroxide Lewis Structure Chemistry Barium Peroxide Png 19x10px Hydrogen Peroxide Area Atom Barium Peroxide Black



Peroxide


Dot Structures Of Molecules Directions



Does Peroxide Ion Have Any Unpaired Electrons Chemistry Stack Exchange



Lecture 7 Polyatomic Ions And Polar Covalent Bonds Flashcards Quizlet



Below Is The Lewis Structure Of The Peroxide O2 2 Ion Co Clutch Prep
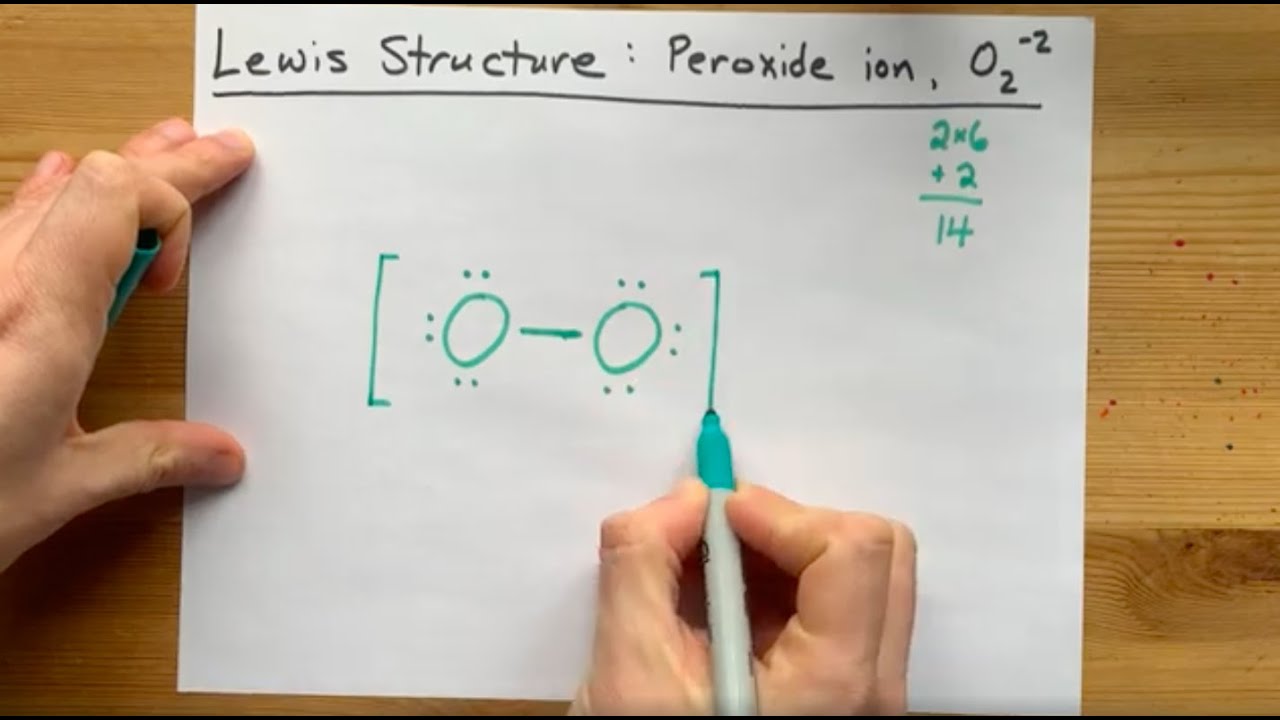


Lewis Structure Of The Peroxide Ion O2 2 Youtube



Solved Below Is The Lewis Structure Of The Peroxide 03 Chegg Com
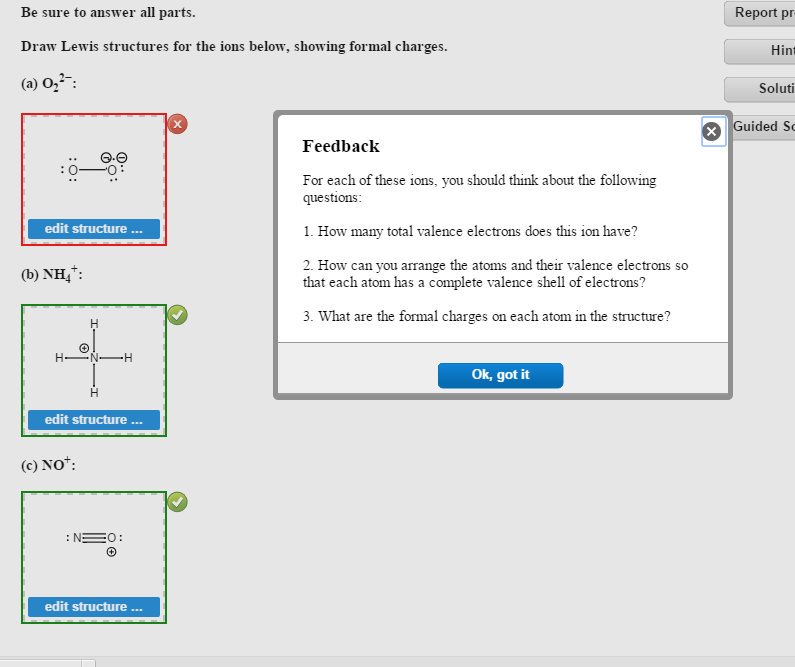


Solved Be Sure To Answer All Parts Draw Lewis Structures Chegg Com



What Is The Difference Between Oxide Peroxide And Superoxide Quora



Solved Question 10 For Each Of The Following Species A Chegg Com



Write Lewis Strucure Of O2 Ion And Find Out Oxidation State Of Each Oxygen Atom What Is The Average Oxidation State Of Oxygen In This Ion


Making Molecules Lewis Structures And Molecular Geometries Annenberg Learner



H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Molecular Geometry Molecular Shapes Intermolecular Force
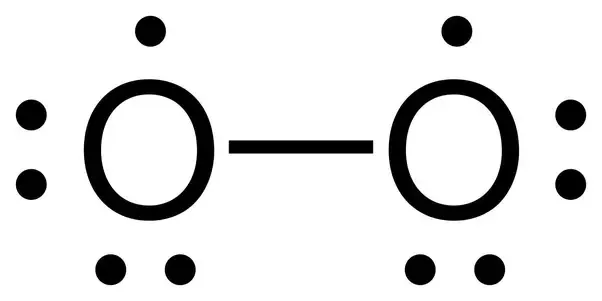


How To Determine The Lewis Dot Structure Of O2 Quora
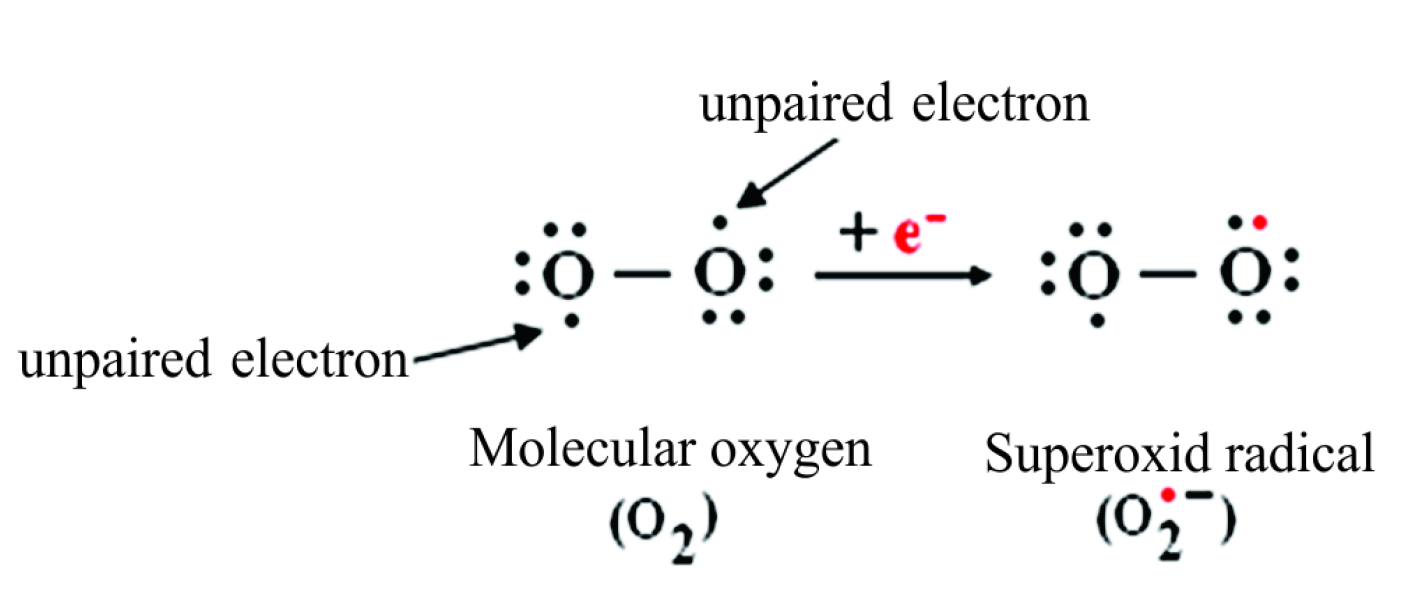


Superoxide Dismutase Therapeutic Targets In Sod Related Pathology



Write The Molecular Orbital Electronic Configuration Of Peroxide A
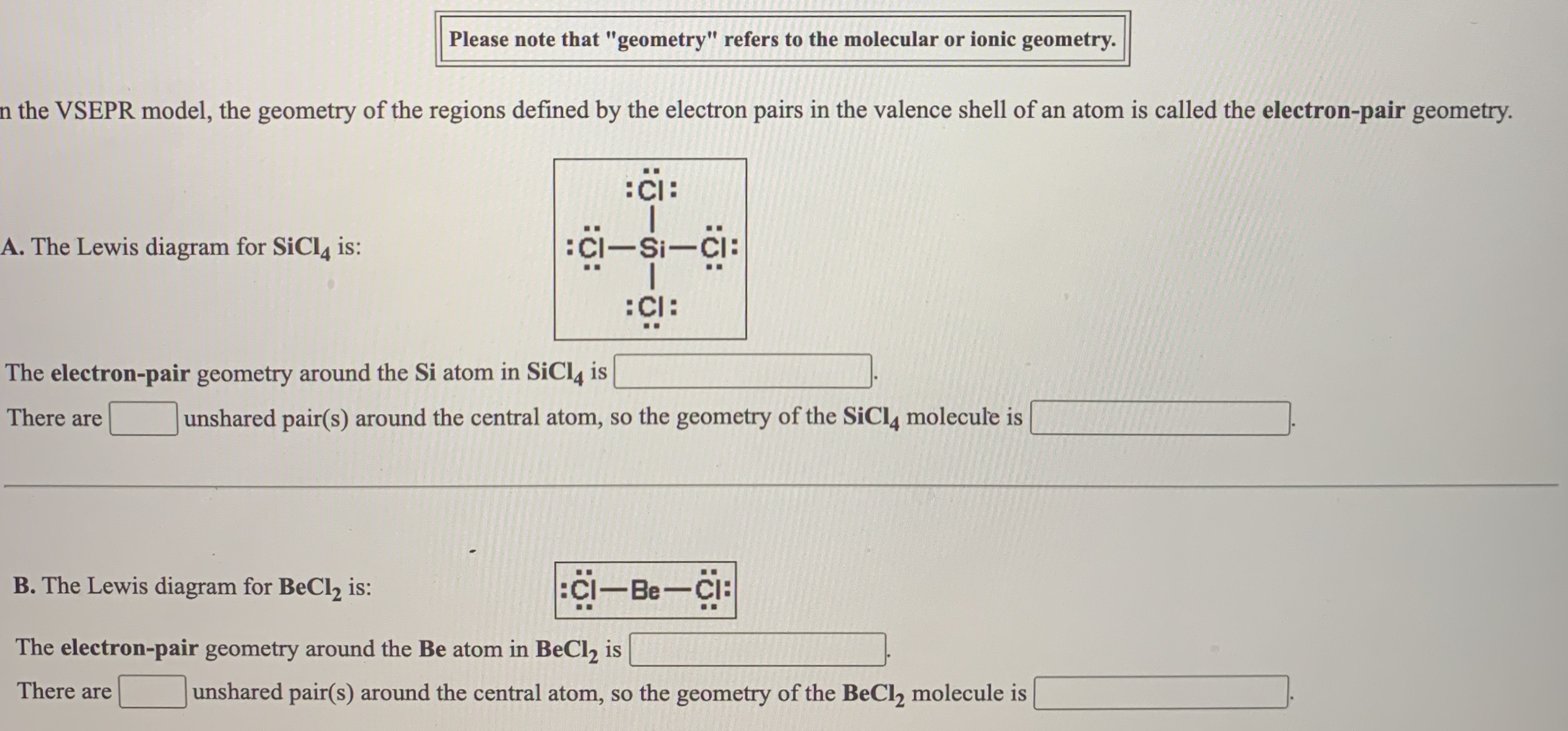


Answered The Vsepr Model The Geometry Of The Bartleby



Toothpastes Containing Sodium Hydrogen Car Clutch Prep



Formal Charges And Resonance Chemistry 2e
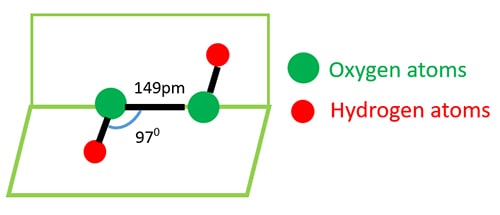


Hydrogen Peroxide Reactions And Physical Properties H2o2



What Is The Lewis Structure For H2o2 Study Com
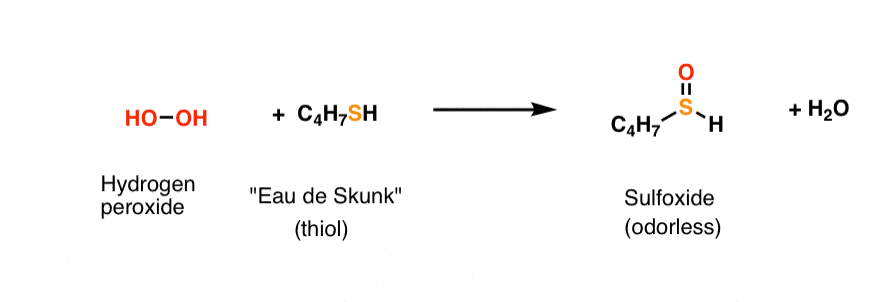


The Marriage May Be Bad But The Divorce Still Costs Money Master Organic Chemistry



H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Chemical Bonding Success In Chemistry



There Are Unpaired Electrons In The Lewis Symbol For An Oxide Ion Brainly Com



How Many Nonbonding Electron Pairs Are The Clutch Prep


Lewisdot



Lewis Dot Structure Of O2 2 Brainly In



0 件のコメント:
コメントを投稿